|
Introduction Antimicrobial resistance among bacterial pathogens has become a serious health threat, and the incidence is rising at an alarming rate. Every year, nearly 2 million patients in American hospitals acquire a nosocomial infection, and more than 70% of the causative pathogens are resistant to at least one of the antibiotics most commonly used to treat them.1 Over 50% of Staphylococcus aureus isolates in intensive care units (ICUs) are resistant to methicillin, oxacillin, or nafcillin (MRSA); 25% of enterococcal isolates are resistant to vancomycin; and 87% of coagulase-negative staphylococci are resistant to methicillin.2 An increasing rate of antibiotic-resistant gram-negative bacilli also has been reported, with the highest incidence seen in Pseudomonas aeruginosa.3 The rise of MRSA has been particularly precipitous and now accounts for about 40% of all nosocomial S aureus infections in the United States.4 Resistant pathogens have been associated with an increased rate of in-hospital mortality, increased morbidity, and a longer length of hospital stay.5 Patients with infections caused by resistant bacteria also are more likely to receive inadequate antimicrobial treatment and incur higher healthcare costs relative to those with antibiotic-susceptible bacterial infections. In US hospitals, annual estimated expenses associated with antibiotic resistance range from $100 million to $30 billion.4 Multiple factors contribute to the escalating emergence of antibiotic resistance. These include the inappropriate use and misuse of antibiotics, especially frequent and unnecessary use of broad-spectrum agents; increasing use of invasive devices; reductions in nursing and other support staff, which raises the likelihood of person-to-person transmission of microorganisms; and increasing numbers of patients with immunosuppression and other similar conditions who are at higher risk for acquiring a drug-resistant infection.6 In addition, prolonged hospitalization appears to predispose patients to either infection or colonization with resistant bacteria. Preventing the Emergence of Resistance Although resistance is not a new phenomenon, the incidence has increased dramatically over the past 2 decades. The development of new drugs has slowed considerably and may be unable to keep pace with the continuing growth of pathogen resistance. Therefore, effective strategies are needed to prevent the continuing emergence of antimicrobial resistance. These include the avoidance of unnecessary antibiotic administration and increasing the effectiveness of prescribed antibiotics, as well as implementing improvements in infection control and optimizing medical practice. The following table summarizes some of the infection control measures for MRSA.
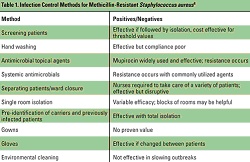
Table 1. Infection Control
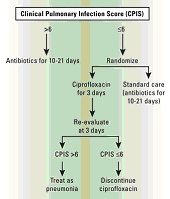
Methods for Methicillin-Resistant Staphylococcus aureus4 Hand Washing Hand washing is widely acknowledged to be the single most important activity for reducing the transmission of infectious agents by both contact and fecal-oral routes.7 Human skin can harbor large quantities and varieties of both infectious and noninfectious microorganisms such as MRSA, which is transmitted primarily on the hands of healthcare workers.8 But compliance with hand hygiene protocols is generally less than optimal. Healthcare workers tend to routinely wash their hands less frequently and for a shorter duration than hospital policy dictates. Pittet et al9 found that compliance with hand washing protocols averaged about 48% among health professionals in a teaching hospital. Noncompliance was higher among physicians, nursing assistants, and other healthcare workers than among nurses. Compliance with hand washing guidelines also was higher in general medical wards than in the ICU and was lower during procedures that carried a high risk for contamination (eg, before intravenous care, 39%; before respiratory care, 18%). Shortages of staffing and higher patient workloads have contributed to poor compliance with hand washing.6,7 Frequent and vigorous hand washing also can damage and break the skin, causing changes in microbial flora and increasing the risk of microorganism transmission.7 Alternative hand washing methods using waterless alcohol-based products have been developed. These are less harsh to the skin than soap and water, do not require access to a sink, and have been shown to be superior to traditional hand washing in reducing bacteria on skin surfaces.10 Voss et al11 found that full compliance with guidelines may be problematic, as workers in the ICU needed a full minute to walk to the sink, wash their hands, and then return to the patient. But whereas between 40 and 80 seconds are needed to wash with soap and water, cleaning the hands with an alcohol-based solution takes only 20 seconds. Thus, compliance may improve by increasing the use of alternative methods. Using Antibiotics Wisely Although there is no single approach that can eliminate drug-resistant pathogens, judicious use of antibiotics is essential for improving patient outcomes, providing better empiric therapy, and minimizing the chances of further antibiotic resistance. Treat Appropriately Up-front Adequate initial antimicrobial treatment is generally defined as an antibiotic regimen that has demonstrated in vitro activity against the pathogen associated with an infection.12 Inadequate treatment has been identified as an important factor in the emergence of infections due to antibiotic-resistant bacteria.13 Increasing clinical evidence suggests that failure to initially treat serious bacterial infections such as hospital-acquired pneumonia and bacteremia with appropriate antimicrobial agents is associated with an increase in patient morbidity and mortality.13 In a study of patients with ventilator-associated pneumonia (VAP), Luna et al 14 found that when adequate antibiotic therapy is initiated very early, even before performing a bronchoscopy, the mortality rate is reduced. But if adequate therapy is delayed until diagnosis is confirmed by bronchoscopy or bronchoalveolar lavage (BAL), then mortality is higher. Even if the antimicrobial regimen is changed to one that is more effective, the mortality rate remains comparable with that of patients who continue to receive inadequate therapy. The necessity of providing adequate initial antimicrobial treatment needs to be balanced with trying to limit the emergence of drug resistance.13 Patients at high risk for infection with antibiotic-resistant bacteria need to be identified. These risk factors include prior antibiotic exposure, use of broad-spectrum antibiotics, prolonged length of hospital stay, prolonged mechanical ventilation, and presence of invasive devices. Healthcare-associated infections also are generally caused by pathogens similar to those responsible for hospital-acquired infections, and identification of these patients is essential to avoiding initial inadequate treatment. Risk factors include receiving home parenteral therapy, hemodialysis treatment at a clinic, recent hospitalization, or residence in a nursing home or long-term care facility. The treatment regimen then needs to be based on individual patient characteristics as well as appropriately using the data presented in an updated hospital-specific or unit-specific antibiogram. Avoid Unnecessary Therapy Antibiotics are an integral part of treating bacterial infections, but their use can be minimized without compromising the patient. De-escalation, giving a shorter course of treatment, and stopping antibiotics when an infection is ruled out all balance the need to provide adequate initial treatment to high-risk patients, while avoiding unnecessary antibiotic use.13 The goal of de-escalation is to first provide an adequate initial antibacterial regimen that will most likely be effective against the bacterial pathogens associated with infection and then to narrow the antibiotic spectrum once the pathogens and their susceptibility profiles are identified.13 Because of the high mortality rates associated with delays in adequate treatment,14 it is important to begin an empiric antimicrobial treatment at the first sign of infection, especially in critically ill patients. But in order to minimize the emergence of drug-resistant infections, the antimicrobial regimen can be narrowed or discontinued completely. This can usually be accomplished within 48 hours of beginning treatment.15 Studies have shown that antibiotic therapy can be shortened in specific patient populations without having an adverse effect on patient outcome.16 Evans et al17 used a computerized guideline to determine antibiotic prescribing in the ICU, and patients with computer-guided therapy had marked reductions in the mean number of days of excessive drug dosage (2.7 vs 5.9) compared with those followed by clinicians not using the program. Clinical outcomes were similar, but length of stay and costs were lower for the patients guided by the computerized anti-infectives–management program. Taking another approach, Singh et al18 found that antibiotic regimens could be both shortened and discontinued in patients with suspected VAP by using the clinical pulmonary infection score (CPIS) as a treatment guideline. Patients with a CPIS of 6 or less, suggesting that they were less likely to have pneumonia, were randomized to receive either standard therapy (choice and duration of antibiotics determined by the clinician) or ciprofloxacin monotherapy with reevaluation at 3 days (Figure 1 below). If the CPIS was still less than or equal to 6, antimicrobial therapy was discontinued. Mortality and length of ICU stay were found to be similar between the 2 groups, despite the shorter duration of therapy. Patients on the shorter regimen also had fewer superinfections with resistant pathogens, including a lower number of infections due to MRSA and P. aeruginosa.
Figure 1. Clinical
Pulmonary Infection Score (CPIS) Disease-Specific Strategies The most common hospital-acquired infections are those of the urinary tract (UTI), surgical site, respiratory tract, and bloodstream.19 In the ICU setting, the presence of an invasive device is overwhelmingly associated with a nosocomial infection; 83% of episodes of nosocomial pneumonia were associated with mechanical ventilation, 97% of UTIs occurred in catheterized patients, and 87% of primary bloodstream infections occurred in patients with a central line. Ventilator-Associated Pneumonia Pneumonia is one of the most commonly reported nosocomial infections and is frequently reported in patients receiving mechanical ventilation. Reported prevalence of VAP ranges from 7% to 65%20,21 and has been associated with increased morbidity, mortality, and a longer length of hospital stay. The causative pathogens vary among healthcare facilities, depending on the population of patients in the ICU, the durations of hospital and ICU stays, and the specific method used to diagnose VAP. A large percentage of VAP cases are increasingly caused by gram-negative bacilli, with S aureus being the predominant gram-positive isolate.21 Etiology also depends on whether the VAP is early or late onset and if the patient has been previously treated with antibiotics.
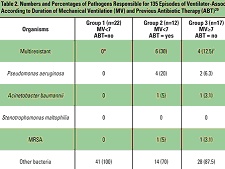
Table 2. Numbers and
Percentages of Pathogens Responsible for 135 Episodes of
Ventilator-Associated Pneumonia Classified According to Duration of
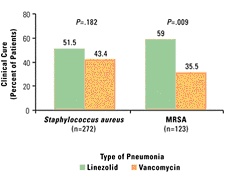
Mechanical Ventilation (MV) and Previous Antibiotic Therapy (ABT)20 Trouillet et al20 found high rates of Haemophilus influenzae, Streptococcus pneumoniae, methicillin-susceptible S aureus (MSSA), and susceptible Enterobacteriaceae in patients with early-onset VAP, particularly in those who had not received prior antimicrobial treatment (Table 2). In contrast, potentially multidrug-resistant pathogens such as P aeruginosa, Acinetobacter baumannii, Stenotrophomonas maltophilia, and MRSA were the predominant organisms in patients with late-onset VAP and who had recently been treated with antibiotics. Risk factors for VAP caused by potentially drug-resistant bacteria were identified as prior antibiotic use, prior use of broad-spectrum agents, and the duration of mechanical ventilation before the onset of VAP. Lower respiratory tract colonization by MRSA and other resistant organisms also closely correlates with the subsequent development of pneumonia.22 Strategies aimed at reducing the burden of bacterial colonization in the aerodigestive tract and decreasing the incidence of aspiration will help prevent the onset of VAP, and similarly, strategies to reverse the trend of resistance also can be implemented. Prolonged antibiotic administration to ICU patients is believed to favor selection and subsequent colonization with resistant pathogens.21 Prophylactic use of parenteral broad-spectrum antibiotics is not recommended as it may increase the risk of VAP caused by multiresistant pathogens, but only delays and does not prevent the occurrence of nosocomial infection.15,21 A gold standard for the diagnosis of VAP does not exist, and as the clinical criteria for pneumonia is nonspecific, inappropriate use of antibiotics for pulmonary infiltrates is common in the ICU. Studies have documented empiric antibiotic treatment for infiltrates in the absence of pneumonia, which in turn raises the prospect of antimicrobial resistance.18 The use of quantitative cultures obtained with BAL, and quantitative assessments that determine the risk of VAP, have reduced prolonged antibiotic use in many patients with suspected infection and lowered both antimicrobial resistance and the cost of antimicrobial treatment.14,22 Bacteremia Bloodstream infections are among the most serious infections acquired by hospitalized patients, and antibiotic resistance appears to have contributed to an increasing degree of inadequate antimicrobial therapy. In a prospective cohort study of clinical outcomes of patients in the ICU, Ibrahim et al5 identified a statistically significant relationship between the rates of inadequate antimicrobial treatment for individual pathogens and their associated rates of mortality. The most common microorganisms identified and their rates of inadequate antimicrobial treatment included vancomycin (100%), Candida species (95.1%), oxacillin-resistant S aureus (32.6%), coagulase-negative staphylococci (21.9%), and P aeruginosa (10%). In a pooled analysis, Cosgrove and colleagues23 also reported that MRSA bacteremia was associated with a significantly higher mortality rate than bacteremia caused by MSSA. A substantial number of cases of bacteremia may be related to intravascular catheters. Over 200,000 bloodstream infections occur in the United States annually, and most are related to an intravascular device, primarily the nontunneled type.24 The risk factors for catheter-related infections vary, according to location of catheter and duration of use and hospital size, unit, or service. Strategies for reducing catheter-related bacteremia include the use of full barrier precautions during catheter insertion; subcutaneous tunneling of short-term catheters inserted in the internal jugular or femoral veins when catheters are not used for drawing blood; the use of antimicrobial-impregnated catheters; povidone-iodine ointment applied to insertion sites of hemodialysis catheters; no routine replacement of central venous catheters; and contamination shields for pulmonary artery catheters.25,26 The risk for infection also has been shown to be higher with catheters inserted into the internal jugular vein, compared with insertion into the subclavian vein.25 In addition, other data indicate that femoral venous catheterization is associated with a greater risk of both infection and thrombosis.27 Urinary Tract Infections Urinary tract infections are the most common nosocomial infection and are often persistent and difficult to treat.19 The overwhelming majority are related to the presence of an indwelling catheter, although other risk factors may play a role. Although UTIs may often be asymptomatic and less expensive to treat than other infections, they can be major reservoirs of antimicrobial-resistant pathogens. Indwelling urinary catheters often have been unnecessarily used or continued longer than necessary. In one study, the initial indication for the placement of an indwelling catheter was found to be unjustified in 21% of 202 medical patients,28 and continued use was found to be unjustified in 47% of 912 patient-days. According to the Centers for Disease Control and Prevention, catheters should only be used when essential and removed when no longer needed.29 If a urinary catheter is deemed necessary, proper aseptic technique, including insertion and maintenance of the catheter and drainage bag, is essential in helping to prevent a UTI.26,29 Surgical Site Infections About 700,000 surgical site infections occur annually in the United States, making them the most common nosocomial infection in surgical patients.30 While the causative organisms may vary depending on the operative site, an increasing proportion of infections are caused by resistant pathogens. Methicillin-resistant S aureus alone now accounts for 28% of all surgical wound infections.31 A study by Gleason et al32 observed that of 441 surgical site infections caused by gram-positive cocci, 248 were antibiotic-resistant strains. Patients with infections caused by the resistant organisms also had a poorer prognosis. Surgical site infections result from a variety of factors, including bacterial contamination of the wound during the procedure, virulence of the contaminating organisms, adjuvant factors within the surgical wound, and patient risk factors. Use of prophylactic antibiotics is generally recommended in curtailing the rates of wound infection, but they need to be administered in a way that does not promote antimicrobial-resistant bacteria. The general consensus is that a dose of an appropriate antibiotic be administered within 60 minutes of the surgical incision to ensure that adequate drug concentrations are present in the serum, tissue, and wound during the entire time that the incision is open and at risk for bacterial contamination.33 Prophylactic antimicrobials should then be discontinued within 24 hours after completion of the procedure, except for cardiothoracic surgery. Nasal colonization with S aureus may be an important independent risk factor for developing a subsequent surgical site infection. Kluytmans and colleagues34 studied the perioperative elimination of nasal carriage using mupirocin nasal ointment. Investigators reported that a significant reduction in the infection rate following cardiothoracic surgery was observed in patients who had been treated with mupirocin compared with those who were not (2.8% vs 7.3%, respectively). Optimization of Antimicrobial Therapy The efficacy of antimicrobial therapy depends upon a number of factors, including the ability to penetrate tissue and the degree to which it binds with protein, which varies considerably among agents. Pharmacokinetic parameters, physicochemical characteristics, and extravascular distribution all play a role in how well an antibiotic will be able to kill or disable the targeted pathogen.35 Physicochemical characteristics may differ between drugs of the same class, such as in the case of fluoroquinolones, which have a high degree of liposolubility. For example, ciprofloxacin is hydrophilic, whereas sparfloxacin is lipophilic, and ofloxacin and lomefloxacin exhibit intermediate liposolubility. However, all agents in this class are similar as far as their tissue diffusion characteristics. After administration, the antimicrobial reaches a stable level in the plasma and eventually binds with plasma proteins. It is then gradually distributed throughout body tissues and fluids. The most appropriate method of antimicrobial administration needs to be determined to promote optimal efficacy. In one study that compared a continuous infusion of ceftazidime with intermittent administration, investigators reported that the continuous infusion was more consistent in keeping serum concentrations of the drug above minimal inhibitory concentrations (MICs) of 4 µg/mL.36 Both methods achieved the same pharmacodynamic parameters, but the continuous infusion used only half the daily dose of intermittent administrations. A continuous infusion offers the potential for lower antimicrobial doses and institutional cost savings. The ability of an antibiotic to concentrate at the infection site is an important factor in determining the success of therapy.35 For example, in UTIs renal tissue concentrations of an antibiotic are a good indicator of efficacy.37 The degree of protein binding is a strong determinant of both the agent's half-life and tissue penetration. Antibiotics with a high affinity to bind with protein tend to have long serum half-lives, whereas low protein-binding agents have short ones.38 Agents that are highly protein bound, such as vancomycin and ceftriaxone, also do not disperse as readily into extravascular sites such as the lungs, bone, and muscle.35,38 Although vancomycin has historically been the treatment of choice for MRSA infections, it may not be the best option for pneumonia. Data from 2 prospective, randomized, double-blind studies that compared linezolid with vancomycin in the treatment of documented pneumonia caused by S aureus, as well as a subset of patients with MRSA pneumonia, indicated that vancomycin was a less effective treatment.39 Patients in the MRSA subset who were treated with linezolid had better survival (80.0% vs 63.5%, P=.03) and clinical cure rates (59.0% vs 35.5%, P<.01) than those who received vancomycin (Figure 2 below). The reason for improved survival in the linezolid group may be due to the poor penetration of vancomycin into the lungs, which has been documented in other studies. Cruciani et al40 evaluated lung tissue penetration of vancomycin in 30 patients undergoing lung resection. Concentrations of vancomycin were undetectable in lung tissue in 1 of 6 patients observed after 6 hours and in 3 of 7 patients at 12 hours. Another investigation that studied vancomycin penetration in epithelial lining fluid indicated that after being administered intravenously at a daily dose of 30 mg/kg, drug levels were undetectable in 6 of 10 patients.41
Figure 2. Type of
Pneumonia Toxin Production The ability to produce toxins is an underlying mechanism harbored by many bacterial pathogens. These include S aureus alpha-toxin, Shiga toxin, cytotoxic necrotizing factor type 1, Escherichia coli heat-stable toxin, botulinum and tetanus neurotoxins, and S aureus toxic shock syndrome toxin. The toxins are not susceptible to antibiotics, and destroying the pathogen before too much toxin is produced often is the only approach to preventing toxic effects, as antitoxins do not exist for many of the bacterial toxins. But at subinhibitory concentrations, antibiotics whose mode of action comprises inhibition of bacterial protein biosynthesis also may modulate the expression of bacterial virulence factors. At sub-MIC concentrations, clindamycin failed to inhibit growth of Streptococcus pyogenes but modified its virulence.42 In a more recent study, subgrowth-inhibitory concentrations of linezolid reduced virulence factors of S aureus and S pyogenes.43 Ohlsen et al44 used a gene fusion model to evaluate the effects of 31 antibiotics on the expression of alpha-toxin (hla) in both MSSA and MRSA isolates at concentrations below the MIC. Glycopeptide antibiotics had virtually no effect on the toxin, whereas erythromycin and several of the aminoglycosides reduced its expression, and fluoroquinolones slightly stimulated hla expression. But the main findings of the study were that beta-lactams strongly induced hla expression; clindamycin almost completely represses hla expression; and methicillin enhances hla production of both MSSA and MRSA. Low concentrations of clindamycin also have been shown to inhibit the expression of staphylococcal toxic shock syndrome toxin. Conclusion Preventing antibiotic-resistant infection requires a multifaceted approach. Judicious use of antimicrobials is key to preventing the emergence of further resistance, as well as implementation of comprehensive infection control programs. Antibiograms need to be updated regularly to keep track of bacterial resistance patterns, and clinicians also need to be aware of which pathogens are primarily responsible for both community-acquired and nosocomial infections. Strategies for preventing infection, such as minimizing the use of invasive devices and reducing bacterial colonization, will not only reduce the rate of infections but also improve patient outcomes, reduce the prevalence of antimicrobial-resistant pathogens, and decrease healthcare-related costs. The antibiotic susceptibility profiles of these pathogens should be routinely available to physicians to guide their selection of antimicrobials, particularly as few new classes of agents are likely to become available for clinical use in the short term. Preventing healthcare-associated infections caused by antimicrobial-resistant pathogens requires a comprehensive approach that includes: (1) preventing infections through the use of vaccines and prophylaxis; (2) minimizing the use of invasive devices; (3) understanding, implementing, and complying with current guideline recommendations for the prevention of infections; and (4) using antimicrobials judiciously. Implementing such a comprehensive program will reduce healthcare-associated infections, reduce the prevalence of antimicrobial-resistant pathogens, reduce healthcare costs, and improve patient outcomes. Finally, there is dire need for continued development of new antibiotics and new technologies to improve their delivery to the infection site. Continued research is needed to identify and develop new and innovative approaches to treating infectious diseases. References
|